Vaccine Therapy: A New Frontier in Type 1 Diabetes Care
Published on 10 Jul, 2024

Type 1 diabetes, once considered a lifelong affliction requiring constant management, has seen remarkable advancements in recent years, particularly in the field of alternative therapies. The traditional management of type 1 diabetes involves daily insulin injections or the use of insulin pumps to maintain blood sugar within a healthy range. While these methods are effective at managing the symptoms and reducing the risk of complications, they do not address the underlying cause of the disease. The emergence of vaccine treatments offers a new avenue of hope for those living with this chronic condition.
In the quest to combat type 1 diabetes (T1D), researchers have explored various vaccine strategies aimed at modulating the immune system's response to pancreatic beta cell antigens. These approaches include antigen-specific vaccines, altered peptide ligands (APLs), recombinant vaccines, live attenuated and DNA-based vaccines, each offering unique mechanisms to target the autoimmune processes underlying the disease.
- Antigen-specific vaccines: These are designed to induce immune tolerance to specific antigens involved in the pathogenesis of T1D. One of the most studied antigens in this context is glutamic acid decarboxylase (GAD), an enzyme found in pancreatic beta cells. Vaccines containing GAD or its fragments aim to stimulate regulatory T cells (Tregs) that suppress the autoimmune response against beta cells.
- Altered peptide ligands: These are synthetic peptides derived from native beta cell antigens but modified to attenuate their immunogenicity. One example is the use of proinsulin-derived peptides with modified amino acid sequences. These modified peptides can bind to MHC molecules and stimulate regulatory T cells, thereby suppressing autoreactive T cells targeting proinsulin.
- Peptide-based vaccines: These consist of short amino acid sequences derived from beta cell antigens, such as insulin or GAD. These peptides are selected based on their ability to elicit regulatory immune responses while avoiding activation of effector T cells that contribute to beta cell destruction.
- DNA-based vaccines: These target specific autoantigens implicated in the pathogenesis of T1D. The vaccines work by delivering DNA sequences encoding the target antigens into host cells, stimulating the production of regulatory T cells that suppress the autoimmune response against pancreatic beta cells.
Clinical Trials
In recent years, significant progress has been made in the development of vaccine treatments for T1D. Clinical trials have focused on refining vaccine formulations, optimizing dosing regimens, and identifying biomarkers to predict treatment response.
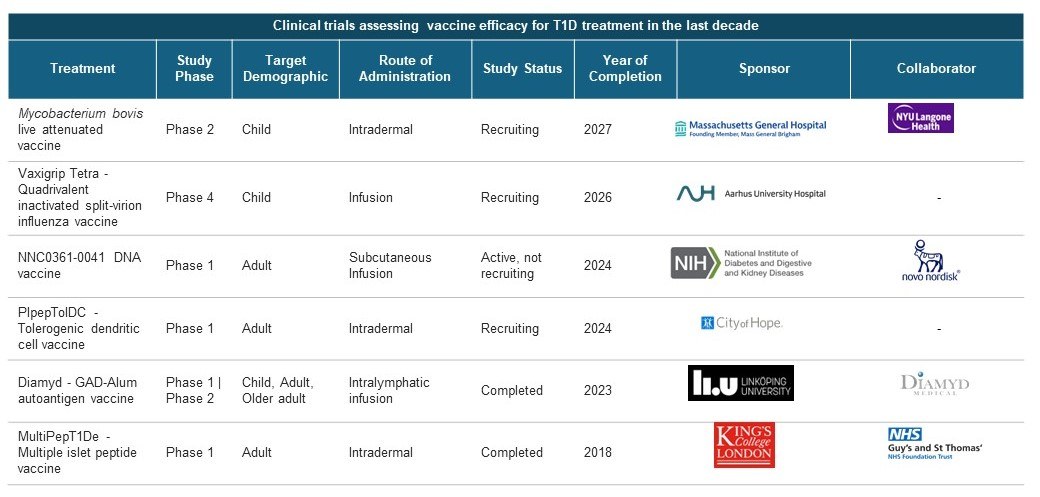
Table 1: Clinical trials assessing vaccine efficacy for T1D treatment in the last decade
Research Hotspots
Over the past decade, approximately 70% of R&D efforts focused on developing T1D vaccines have been led by corporations, academia, and research organizations based in countries such as the US, China, Sweden, the UK, Germany, Finland, and Canada.
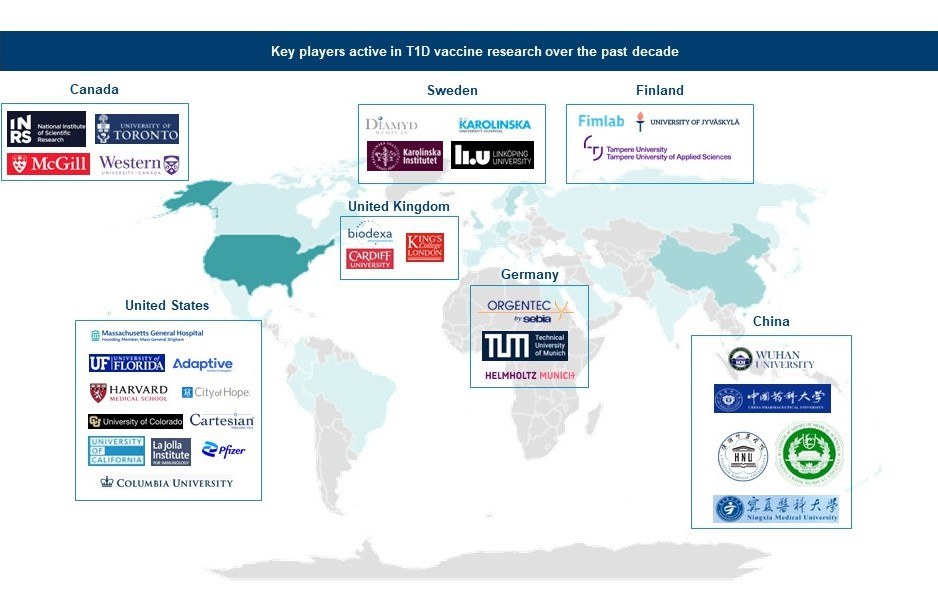
Figure 1: Key players active in T1D vaccine research over the past decade
The majority, approximately 85%, of patent applications pertaining to T1D vaccines originate from players based in China, the US, France, and Japan.
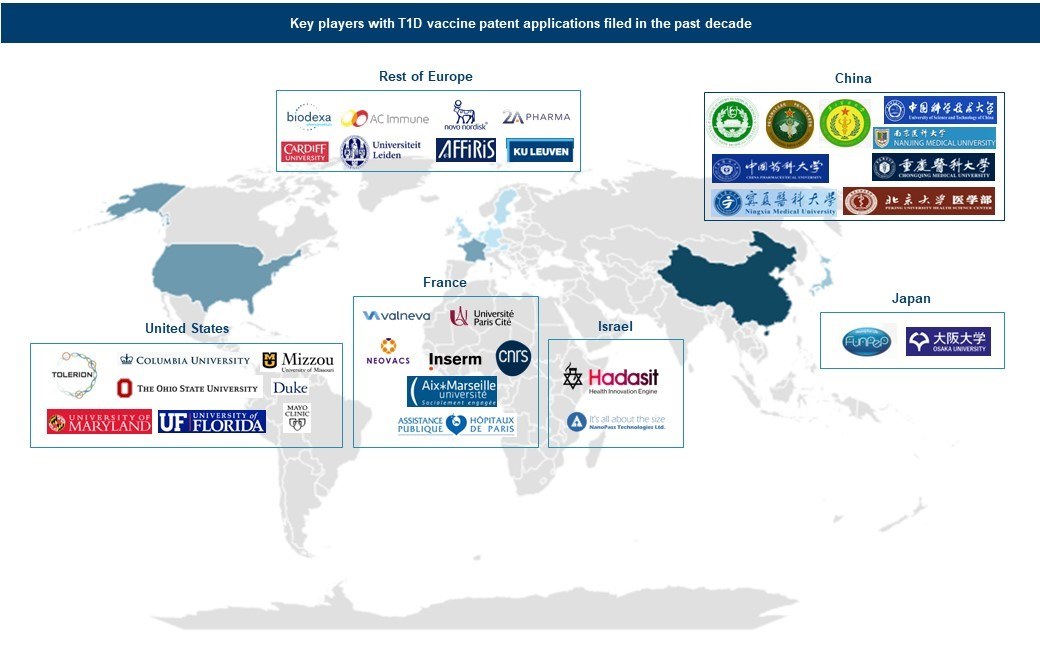
Figure 2: Key Players with T1D vaccine patent applications filed in past decade
Market Snapshot
Corporate efforts toward T1D vaccines have increased in recent years, highlighting a growing market interest. Novo Nordisk, in collaboration with the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), initiated a clinical trial in 2020 to evaluate the efficacy of its DNA vaccine (NNC0361-0041). Similarly, Adaptive Biotechnologies partnered with Massachusetts General Hospital and Harvard Medical School in 2022 to explore the therapeutic potential of the BCG vaccine for autoimmune conditions such as T1D. Furthermore, Diamyd Medical made considerable progress by entering into a partnership with DiaUnion in 2023 for the DiaPrecise trial, assessing the feasibility, immune response, and safety of intralymphatic Diamyd injections in children at risk of developing T1D. These collaborative efforts underscore a promising trajectory in T1D vaccine R&D.
Regulatory Front
Amid the dynamic landscape of T1D R&D, remarkable strides have been made in vaccine innovation and regulatory recognition. In 2024, for example, the FDA granted Fast Track designation to Diamyd Medical's Diamyd® vaccine, targeting recently diagnosed stage 3 T1D patients with the HLA DR3-DQ2 genotype. This decision was based on phase 2b DIAGNODE-2 study data, showing improved glycemic control and reduced mealtime hyperglycemic events.
Challenges
Despite progress in vaccine therapy for T1D, several challenges remain:
- Disease complexity: Heterogeneity of the disease, with variations in immune response and progression among individuals is the key challenge.
- Cost: High costs encompassing expenses related to research, development, clinical trials, production, and accessibility hinder affordability and widespread adoption.
- Specificity: Ensuring vaccine specificity for autoreactive T cells is crucial to avoid unintended immune effects or infection vulnerability.
- Regulatory and clinical challenges: Successfully navigating the regulatory approval process and conducting large-scale clinical trials to demonstrate safety and efficacy are resource-intensive and time-consuming tasks for vaccine therapies.
Future Perspective
The convergence of new technologies and a deeper understanding of the complex etiology of T1D offers a hopeful outlook for personalized treatments. Recent advancements in vaccine therapy for T1D signal exciting prospects for improved treatment modalities. Researchers are exploring innovative strategies such as X-idiotype vaccines that harness specific antibodies mimicking antigens to redirect the immune response toward self-tolerance. Reverse vaccine technology is another promising development, reprogramming the immune system to dampen autoimmune reactions instead of stimulating them. These advancements, coupled with exploration of novel delivery routes like intradermal and intra-lymphatic administration, signify a multifaceted approach toward tailored T1D management and intervention.

